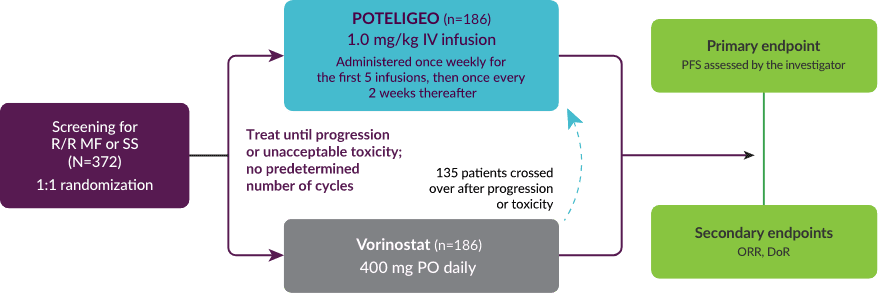
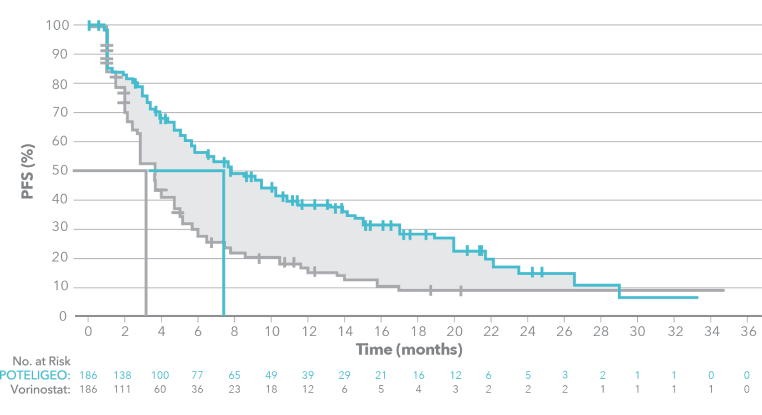
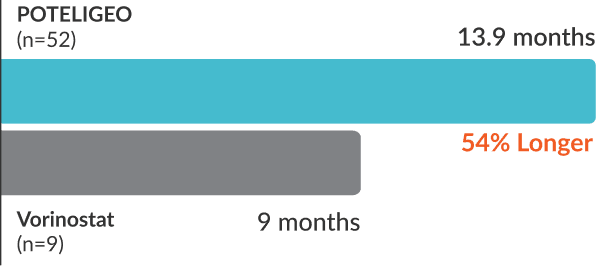
POTELIGEO more than doubled median progression-free survival vs vorinostat1
Primary endpoint:
PFS (Investigator-assessed)
Vorinostat
(n=186)
POTELIGEO
(n=186)
reduction in the risk of disease progression HR=0.53 (P<0.001) 95% Cl: 0.41, 0.69
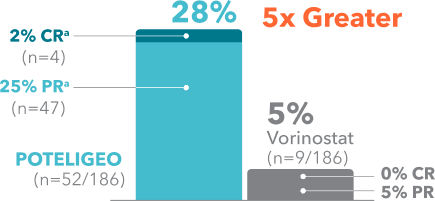
POTELIGEO achieved greater ORR and longer DoR vs vorinostat1
Secondary endpoint: ORR
(P<0.001)
| Overall response was defined as2,3 | ||||
| ≥50% improvement in skin | + | ≥50% improvement in at least 1 other involved compartment | + | No progression of disease in any compartment |
Overall response was defined as2,3
≥50% improvement in skin
+
≥50% improvement in at least 1 other involved compartment
+
No progression of disease in any compartment
Secondary endpoint: DoR (median)1
- ORR was based on global composite response score. Responses in blood and skin must have persisted for at least 4 weeks to be considered confirmed and were evaluated every 4 weeks for the first year. Responses in lymph nodes, in visceral disease, and overall were evaluated every 8 weeks for the first year.1
- Median dose intensity was >95% for both treatment arms.2
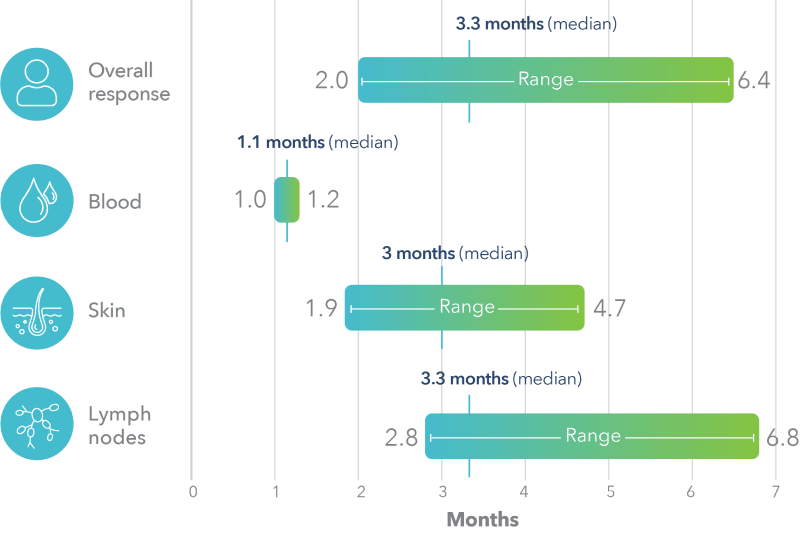
POTELIGEO had a median time to response of about 1 month in blood and 3 months in skin2,b-g
Time to response was measured based on a post hoc analysis; a finding from the post hoc analysis cannot be used to demonstrate differences between treatments and may not be applicable to all patients initiating POTELIGEO.
Post hoc analysis: time to response
- aRepresents complete or partial best overall response rate. Best overall response was defined as the best response from the start of treatment until disease progression/recurrence or end of treatment.3
- bResponse was defined by international response criteria for Mycosis Fungoides and Sézary Syndrome.4
- cResponses in skin and blood must have persisted for ≥4 weeks to be confirmed and were evaluated every 4 weeks during treatment.1,3
- dResponses in lymph nodes were evaluated at 4 weeks, then every 8 weeks for the first year, and every 16 weeks thereafter.2
- eResponse in skin defined as ≥50% clearance of skin disease without new tumors, evaluated using the modified Severity-Weighted Assessment Tool (mSWAT).3
- fResponse in blood defined as >50% decrease in high blood tumor burden (B2), assessed by central flow cytometry.3
- gResponse in lymph nodes defined as cumulative reduction ≥50% of measurable disease of each abnormal lymph node and no new abnormal lymph nodes, evaluated by computed tomography (CT) scans.3
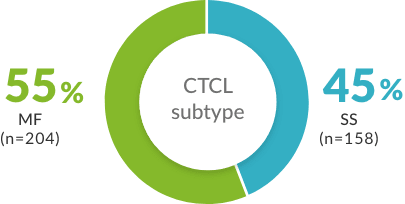
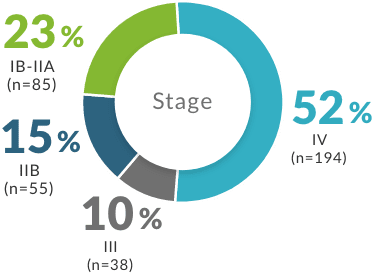
- CCR4=C-C chemokine receptor type 4; CNS=central nervous system; CR=complete response; CTCL=cutaneous T-cell lymphoma; DoR=duration of response; ORR=overall response rate; PFS=progression-free survival; PO=by mouth; PR=partial response; R/R=relapsed or refractory
POTELIGEO has a consistent safety profile with up to 5 years of data7
- POTELIGEO [package insert]. Kyowa Kirin Inc., Princeton, NJ USA.
- Kim YH, Bagot M, Pinter-Brown L, et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018;19(9):1192-1204.
- Kim YH, Bagot M, Pinter-Brown L, et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018;19(9):1192-1204. Supplementary appendix published online August 9, 2018.
- Data on file. Kyowa Kirin Inc., Princeton, NJ USA.
- Cowan RA, Scarisbrick JJ, Zinzani PL, et al. Efficacy and safety of mogamulizumab by patient baseline blood tumour burden: a post hoc analysis of the MAVORIC trial. J Eur Acad Dermatol Venereol. 2021;35(11):2225-2238.
- Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29(18):2598-2607.
- Bagot M, Dalle S, Sokol L, et al. Long-term disease control and safety with the anti-CCR4 antibody mogamulizumab: post-hoc analyses from the MAVORIC trial of patients with previously treated cutaneous T-cell lymphoma. Dermatol Ther. 2022;35(8):e15634.