CCR4 is an important target for Mycosis Fungoides and
Sézary Syndrome1-5
CCR4 receptors are often highly expressed on the surface of malignant T cells in patients with Mycosis Fungoides (MF) and Sézary Syndrome (SS), regardless of stage or skin and blood involvement.1-3
- CCR4 plays a pivotal role in the homing of malignant T cells to the skin in Mycosis Fungoides lesions4,5
- High levels of CCR4 expression may be associated with an increased risk of disease progression4,5
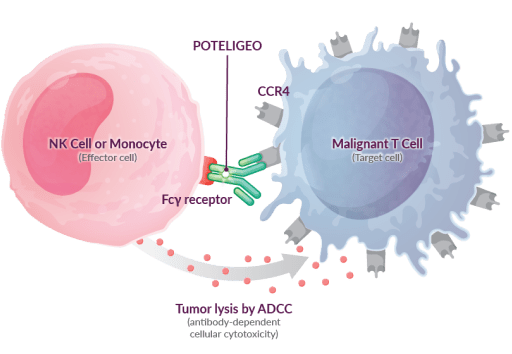
POTELIGEO is a nonchemotherapeutic biologic therapy that targets CCR4+ T cells for destruction via antibody-dependent cellular cytotoxicity (ADCC)6-8
POTELIGEO is not an immunosuppressant.6,9
POTELIGEO was engineered using POTELLIGENT® technology, which has been shown to increase binding affinity and has demonstrated up to 100x increase in ADCC activity compared with conventional antibodies.10
MOA was determined through in vitro studies. No direct link can be made between MOA and efficacy.6,8
These studies demonstrate that POTELIGEO binds to CCR4, which targets and marks them for destruction by effector cells, or natural killer cells.6,8
- This results in depletion of the target cells, including malignant T cells
There is no requirement to test for CCR4 prior to treating with POTELIGEO6

- CCR4=C-C chemokine receptor type 4; CTCL=cutaneous T-cell lymphoma; MOA=mechanism of action; NCCN=National Comprehensive Cancer Network®; NK=natural killer
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) included mogamulizumab (POTELIGEO) as a preferred treatment option for CTCL11
recommendations
- Ferenczi K, Fuhlbrigge RC, Pinkus J, Pinkus GS, Kupper TS. Increased CCR4 expression in cutaneous T cell lymphoma. J Invest Dermatol. 2002;119(6):1405-1410.
- Kakinuma T, Sugaya M, Nakamura K, et al. Thymus and activation-regulated chemokine (TARC/CCL17) in mycosis fungoides: serum TARC levels reflect the disease activity of mycosis fungoides. J Am Acad Dermatol. 2003;48(1):23-30.
- Kallinich T, Muche JM, Qin S, Sterry W, Audring H, Kroczek RA. Chemokine receptor expression on neoplastic and reactive T cells in the skin at different stages of mycosis fungoides. J Invest Dermatol. 2003;121(5):1045-1052.
- Campbell JJ, Clark RA, Watanabe R, Kupper TS. Sézary syndrome and mycosis fungoides arise from distinct T-cell subsets: a biologic rationale for their distinct clinical behaviors. Blood. 2010;116(5):767-771.
- Nicolay JP, Albrecht JD, Alberti-Violetti S, Berti E. CCR4 in cutaneous T-cell lymphoma: therapeutic targeting of a pathogenic driver. Eur J Immunol. 2021;51(7):1660-1671.
- POTELIGEO [package insert]. Kyowa Kirin Inc., Princeton, NJ USA.
- Ishida T, Iida S, Akatsuka Y, et al. The CC chemokine receptor 4 as a novel specific molecular target for immunotherapy in adult T-cell leukemia/lymphoma. Clin Cancer Res. 2004;10(22):7529-7539.
- Duvic M, Evans M, Wang C. Mogamulizumab for the treatment of cutaneous T-cell lymphoma: recent advances and clinical potential. Ther Adv Hematol. 2016;7(3):171-174.
- National Cancer Institute. NCI dictionary of cancer terms. Accessed November 15, 2024. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/immunosuppressive-agent
- Matsushita T. Engineered therapeutic antibodies with enhanced effector functions: clinical application of the Potelligent® technology. Korean J Hematol. 2011;46(3):148-150.
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Primary Cutaneous Lymphomas V.1.2025. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed February 11, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
