Sézary Syndrome is an aggressive cutaneous
T-cell lymphoma (CTCL) subtype1
Typical Sézary Syndrome presentation on the skin is highly erythrodermic; Sézary Syndrome also involves a high blood tumor burden and generalized lymphadenopathy2
| Blood involvement in Sézary Syndrome: | |
|---|---|
Is usually classified as B2
|
Is associated with poorer prognosis/shorter survival for both Mycosis Fungoides and Sézary Syndrome3
|
Two studies found a significant difference in median overall survival for B0 and B23,5
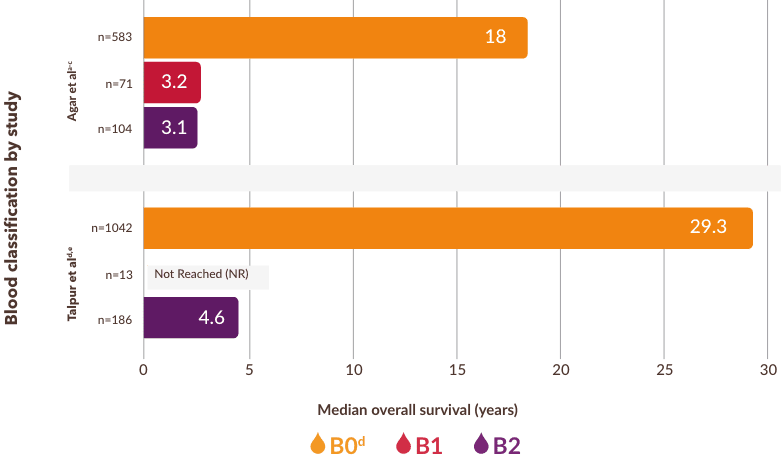
- In a third study, Scarisbrick et al also found a trend toward worse survival with increasing blood involvement6
National Comprehensive Cancer Network® (NCCN®) currently defines blood classification based on the following8,9:
BLOOD CLASSIFICATION
-

-
<250/μL
CD4+CD26- or CD4+CD7-
cells by flow cytometry
-

-
250/μL to <1000/μL
CD4+CD26- or CD4+CD7-
cells by flow cytometry
-

-
≥1000/μL
CD4+CD7- or CD4+CD26-
cells or other aberrant phenotype in the presence of a relevant T-cell clone in blood
- NCCN=National Comprehensive Cancer Network
- aBlood classification defined in the Agar study: B0=No blood involvement (≤5% Sézary cells); B0a=clone-negative; B0b=clone-positive; B1=low tumor burden (>5% Sézary cells but not meeting B2 criteria); B2=high tumor burden (≥1000/μL Sézary cells with positive clone).3,7
- bRetrospective analysis of patients with Mycosis Fungoides and Sézary Syndrome from the ICARSIS CTCL registry (1980-2009).3
- cIn the Agar study, B0 represents those patients without T-cell receptor (TCR) data.3
- dBlood classification defined in the Talpur study: B0=No blood involvement (<500 Sézary cells/μL); B1=low tumor burden (>500 and <1000 cells/μL); B2=high tumor burden (>1000 cells/μL or >35% of lymphocytes as CD4+CD26– or CD4+CD7– cells).5
- eIn the Talpur study, median survival for patients with B1 levels of blood involvement was not reached due to limited number of patients.5
CTCL Expert Perspectives: Patient Communication
Lauren C. Pinter-Brown, MD, clinical professor of Hematology/Oncology at University of California, Irvine, discusses how to communicate with patients who have Mycosis Fungoides or Sézary Syndrome on topics including disease state, prognosis, blood involvement, staging and prescribing.
Blood testing via flow cytometry for suspected Sézary Syndrome is important at diagnosis and throughout treatment to monitor disease burden and response to treatment10
Explore POTELIGEO MOA- Kim YH, Bagot M, Pinter-Brown L, et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018;19(9):1192-1204.
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133(16):1703-1714.
- Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28(31):4730-4739.
- Scarisbrick JJ, Hodak E, Bagot M, et al. Blood classification and blood response criteria in mycosis fungoides and Sézary syndrome using flow cytometry: recommendations from the EORTC cutaneous lymphoma task force. Eur J Cancer. 2018;93:47-56.
- Talpur R, Singh L, Daulat S, et al. Long-term outcomes of 1,263 patients with mycosis fungoides and Sézary syndrome from 1982 to 2009. Clin Cancer Res. 2012;18(18):5051-5060.
- Scarisbrick JJ, Princes HM, Vermeer MH, et al. Cutaneous lymphoma international consortium study of outcome in advanced stages of mycosis fungoides and Sézary syndrome: effect of specific prognostic markers on survival and development of a prognostic model. J Clin Oncol. 2015;33(32):3766-3773.
- Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification of mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110(6):1713-1722.
- Olsen EA, Whittaker S, Willemze R, et al. Primary cutaneous lymphoma: recommendations for clinical trial design and staging update from the ISCL, USCLC, and EORTC. Blood. 2022;140:419-437
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Primary Cutaneous Lymphomas V.1.2023. © National Comprehensive Cancer Network, Inc. 2023. All rights reserved. Accessed July 13, 2023. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Vermeer MH, Moins-Teisserenc H, Bagot M, Quaglino P, Whittaker S. Flow cytometry for the assessment of blood tumour burden in cutaneous T-cell lymphoma: towards a standardized approach. Br J Dermatol. 2022;187(1):21-28.
