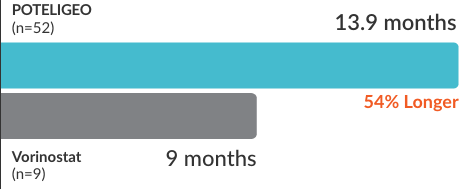
POTELIGEO achieved greater ORR and longer DoR vs vorinostat1
Secondary endpoint: ORR (P<0.001)
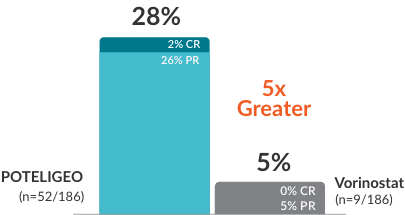
Secondary endpoint: DoR (median)
Overall response was defined as2,3
≥50% improvement in skin
+
≥50% improvement in at least 1 other involved compartment
+
No progression of disease in any compartment
Median time to overall response4,a: (post hoc analysis)
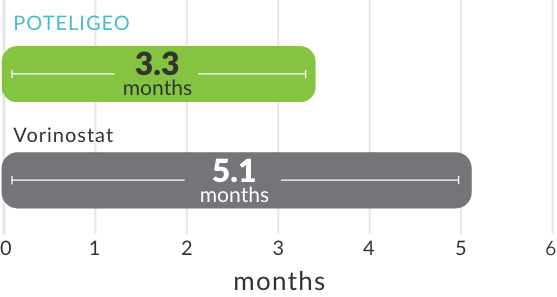
- aTime to response was measured based on a post hoc analysis; a finding from the post hoc analysis cannot be used to demonstrate differences between treatments and may not be applicable to all patients initiating POTELIGEO.
- In the first year of treatment, overall response was assessed at the end of Cycle 1 and every 8 weeks thereafter (Cycles 3, 5, 7, etc). After the first year, overall response was assessed every 16 weeks (Cycles 17, 21, 25, etc). Responses from baseline had to be demonstrated at 2 consecutive assessments for patient to be considered a responder.2
- Median dose intensity was >95% for both treatment arms.2
- CR=complete response; PR=partial response
See the latest blood response data from the MAVORIC post hoc analysis
Watch the POTELIGEO Efficacy in Blood videoIn a post hoc analysis, POTELIGEO achieved significantly greater overall response rate by blood classification vs vorinostat5
Investigator-assessed ORR by blood classificationb
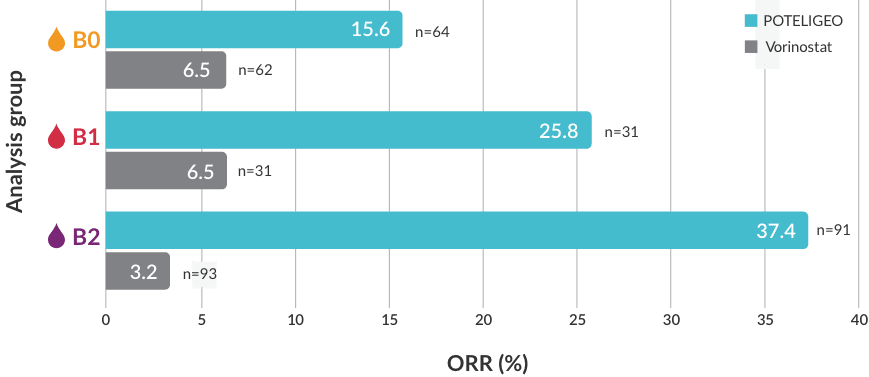
BLOOD CLASSIFICATION
-

-
<15%
CD4+CD26- or CD4+CD7- cells by flow cytometry
-

-
≥15%
CD4+CD26- or CD4+CD7- cells by flow cytometry
-

-
≥1000/μL Sézary cells with positive clone
or 1 of the following:- CD4:CD8 ratio ≥10,
- 40% CD4+CD7- cells, or
- ≥30% CD4+CD26- cells
bORR by blood classification was measured based on a post hoc analysis; a finding from the post hoc analysis cannot be used to demonstrate differences between treatments and may not be applicable to all patients initiating POTELIGEO.
POTELIGEO has a consistent safety profile with up to 5 years of data6
View safety profile- POTELIGEO [package insert]. Kyowa Kirin Inc., Princeton, NJ USA.
- Kim YH, Bagot M, Pinter-Brown L, et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018;19(9):1192-1204.
- Kim YH, Bagot M, Pinter-Brown L, et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018;19(9):1192-1204. Supplementary appendix published online August 9, 2018. doi.org/10.1016/S1470-2045(18)30379-6
- Data on file. Kyowa Kirin Inc., Princeton, NJ USA.
- Cowan R, Scarisbrick JJ, Zinzani PL, et al. Efficacy and safety of mogamulizumab by patient baseline blood tumour burden: a post hoc analysis of the MAVORIC trial. J Eur Acad Dermatol Venereol. 2021;35(11):2225-2238.
- Kim Y, Bagot M, Zinzani PL, et al. Safety of mogamulizumab in mycosis fungoides and Sézary syndrome: final results from the phase 3 MAVORIC study. Blood. 2019;134(suppl):5300[abstract].