Dosing modifications for adverse reactions1
Dermatologic toxicity*
- Monitor patients for rash throughout the treatment course
- Rash (drug eruption) may be visually indistinguishable from disease progression
- Consider skin biopsy to help make a differential diagnosis
- *Grades are based on CTCAE v.50.
- aLess than 1% of all POTELIGEO-treated patients in clinical trials experienced grade 4 skin adverse reactions; SJS occurred in <1% of patients.1
- SJS=Stevens-Johnson syndrome; TEN=toxic epidermal necrolysis
Infusion reactions
Common signs of infusion reactions
chills | nausea | fever | tachycardia | rigors | headache | vomiting
Use in special populations
Pharmacokinetics
No clinically significant changes in the pharmacokinetics of POTELIGEO were observed based on age, renal impairment, or mild/moderate hepatic impairment. As a result, no corresponding dose modifications are required.
Geriatric use
- In MAVORIC, 51% of patients treated with POTELIGEO were 65 years of age or older (range: 25 to 101)
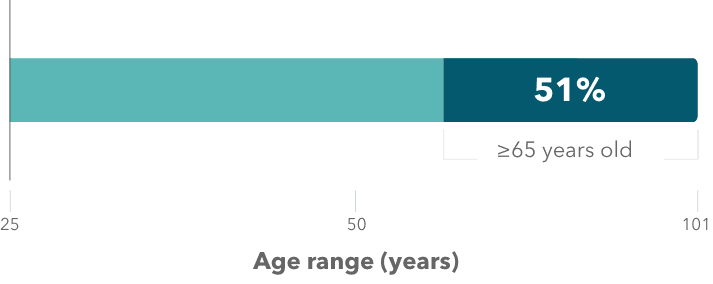
- No overall differences in effectiveness were observed between patients 65 or older and younger patients
- Patients 65 or older had a greater number of grade 3 or higher adverse reactions than patients under 65, but the difference was not statistically significant
Watch leading experts discuss additional treatment and management considerations as part of our CTCL Expert Perspectives video series
Watch the video series- POTELIGEO [package insert]. Kyowa Kirin Inc., Princeton, NJ USA.



